Introduction
If you’ve been diagnosed with chronic kidney disease (CKD), you’ve likely heard about creatinine, eGFR, and potassium levels. But there’s a crucial enzyme that most doctors and even medical textbooks still aren’t discussing: renalase. Discovered only in 2005, this remarkable protein plays a central role in kidney health, blood pressure regulation, and the body’s stress response. Understanding renalase may be the key to understanding why kidney disease creates such a cascade of cardiovascular and metabolic problems, and why stress seems to accelerate kidney decline.
What Is Renalase?
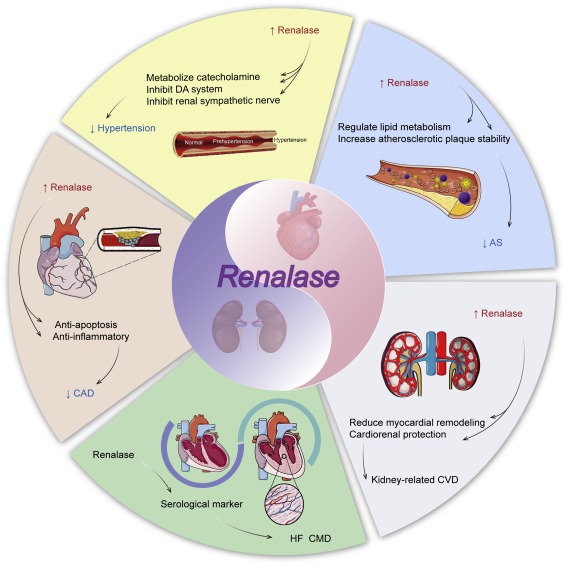
Renalase is an enzyme, a specialized protein that your body produces primarily in the kidneys. Its main job is to break down catecholamines, which are stress hormones like epinephrine (adrenaline), norepinephrine, and dopamine. Think of renalase as your body’s cleanup crew for stress hormones. When these catecholamines have done their job of responding to stress, renalase degrades them so they can be eliminated from your body.
But renalase does far more than just clean up stress hormones. It functions as an NADH oxidase and acts as a cytoprotective signaling molecule, meaning it protects your cells from damage and helps coordinate important cellular functions. This enzyme is predominantly active in the proximal tubule cells of the kidney, which are the workhorses responsible for filtering your blood and reabsorbing essential nutrients.
The Role of Renalase in the Body
Throughout your body, renalase serves several critical functions:
Stress Response Modulation: By degrading catecholamines, renalase prevents the sympathetic nervous system (your “fight or flight” response) from staying in overdrive. When renalase levels are healthy, your body can respond appropriately to stress and then return to a calm, balanced state.
Blood Pressure Regulation: The breakdown of catecholamines directly affects blood vessel tone. When epinephrine and norepinephrine accumulate because renalase is low, blood vessels constrict, driving blood pressure upward. Renalase helps prevent this chronic vasoconstriction.
Cardiovascular Protection: Renalase protects against catecholamine-driven oxidative bursts essentially preventing stress hormones from creating excessive free radicals that damage your heart and blood vessels.
Mitochondrial Health: Your mitochondria are the energy powerhouses of your cells, and kidneys are packed with them because filtering blood requires enormous energy. Renalase helps protect these mitochondria from stress-induced damage.
Renalase’s Crucial Role Within the Kidney
In the kidneys specifically, renalase is absolutely essential for maintaining healthy function. The kidneys are not just filters, they’re sophisticated regulators of blood pressure, electrolytes, and hormones. Renalase plays a central role in several kidney functions:
Tubular Protection: The kidney tubules, particularly the proximal tubules, contain the highest concentrations of renalase. These tubules are responsible for the intricate work of deciding what to keep and what to excrete. Renalase protects these tubule cells from apoptosis (premature cell death) and helps prevent tubulointerstitial fibrosis, the scarring that makes kidney damage irreversible.
Sodium and Water Balance: By modulating the effects of aldosterone and the renin-angiotensin system, renalase indirectly affects how your kidneys handle sodium and water reabsorption, which in turn affects blood pressure and fluid balance.
pH Balance: Through its effects on the tubules and the excretion of various substances, renalase helps maintain proper acid-base balance in your blood.
Partnership with Klotho: Renalase works in concert with another crucial kidney protein called Klotho. Both are suppressed early in kidney tubule stress often long before creatinine levels change on your lab work. When both renalase and Klotho are low, the risk of tubulointerstitial fibrosis increases dramatically.
The Stress-Kidney Connection: How Renalase Links Them Together
One of the most fascinating, and clinically important, aspects of renalase is how it explains the stress-kidney connection. Many people with kidney disease notice that stressful life events seem to accelerate their decline. This isn’t psychological; it’s physiological.
Chronic Sympathetic Excitation: Chronic sympathetic nervous system overactivity is a hallmark of chronic kidney disease. When your kidneys are damaged, renalase production drops. With less renalase available to break down stress hormones, catecholamines accumulate, creating a state of chronic sympathetic activation. This feels like anxiety, hypervigilance, or being “wired and tired,” but it’s not a mental health issue it’s a physical consequence of impaired catecholamine clearance.
The Vicious Cycle: Low renalase leads to increased catecholamines, which causes more kidney stress and damage, which further lowers renalase. Meanwhile, the elevated catecholamines trigger renin release, activating the renin-angiotensin-aldosterone system (RAAS), which causes blood vessel constriction, decreased blood flow to the kidneys, and even more reduction in renalase production. It’s a downward spiral.
The Non-Dipper Phenomenon: Many people with kidney disease experience reversed circadian rhythms they’re wired at night and exhausted during the day. This “non-dipper” pattern (where blood pressure doesn’t drop at night as it should) correlates with blunted diurnal cortisol decline. Your cortisol and blood pressure should naturally decrease at night, but when renalase is low and catecholamines are elevated, this normal pattern is disrupted.
Tests and Symptoms That Suggest Low Renalase Levels
Unfortunately, renalase itself cannot currently be tested in standard clinical laboratories. However, we can identify when renalase is likely low by looking at downstream markers and clinical symptoms:
Laboratory Markers:
- Elevated plasma or urinary catecholamines (epinephrine, norepinephrine)
- Elevated aldosterone or renin levels, even if they’re just “borderline high”
- Rising FGF-23 (fibroblast growth factor 23)
- Declining eGFR
- Rising inflammatory markers (CRP, cytokines)
- Elevated markers of tubular stress (β2-microglobulin, retinol-binding protein, Kim-1)
- Changes in phosphorus handling
Clinical Symptoms:
- Hypertension, particularly if it’s difficult to control or sympathetically driven
- POTS (Postural Orthostatic Tachycardia Syndrome) symptoms
- Anxiety that doesn’t respond well to conventional treatment
- Sleep disturbances, particularly difficulty falling asleep or staying asleep
- Feeling “wired but tired”
- Blood pressure that rises at night instead of dropping
- Heart palpitations or awareness of rapid heartbeat
- Stress triggering noticeable declines in kidney function
- Frailty or muscle weakness (especially combined with low potassium)
It’s crucial to understand that when you have kidney disease, symptoms that might be dismissed as “just anxiety” or “just stress” may actually reflect your body’s inability to clear catecholamines properly. This distinction is vital, you don’t need counseling for anxiety; you need support for your kidney’s impaired detoxification capacity.
The Link Between Low Renalase, CKD, Hypertension, and Inflammation
Low renalase creates a perfect storm for cardiovascular and metabolic complications:
Hypertension: When renalase is decreased, catecholamines accumulate, causing vasoconstriction and stimulating aldosterone release. Aldosterone increases sodium reabsorption in the kidney tubules, leading to fluid retention and vascular stiffness. Both mechanisms drive blood pressure higher. Even when aldosterone and renin levels appear “normal” on lab work, the chronic sympathetic activation creates SNS-driven hypertension.
Left Ventricular Hypertrophy: The combination of hypertension and increased catecholamines forces the heart to work harder, leading to thickening of the heart muscle (left ventricular hypertrophy). This is one reason cardiovascular disease is the leading cause of death in kidney disease.
Oxidative Stress and Inflammation: Low renalase means increased catecholamine exposure, which triggers oxidative bursts the production of reactive oxygen species (ROS) that damage cells. This oxidative stress occurs in both kidney tubules and blood vessels. Aldosterone also independently induces ROS in mitochondria. The result is a chronic inflammatory state with elevated pro-inflammatory cytokines.
FGF-23 and the Mineral Metabolism Cascade: When renalase decreases and sympathetic activation increases, renin goes up, affecting vitamin D metabolism and triggering rises in FGF-23. FGF-23 is initially adaptive trying to protect you from phosphorus toxicity but becomes maladaptive when chronically elevated, causing cardiac toxicity and further suppressing Klotho. Low renalase makes your body more sensitive to FGF-23’s toxic effects on the heart.
Endothelial Dysfunction: Catecholamines interfere with nitric oxide production, the molecule responsible for keeping blood vessels relaxed and flexible. This endothelial dysfunction contributes to both hypertension and reduced kidney blood flow.
Fibrosis: Aldosterone induces fibrosis through mineralocorticoid receptor activation, while low renalase fails to suppress pro-fibrotic cytokines. The combination accelerates the scarring of kidney tissue, making damage harder to reverse.
Natural Ways to Support Healthy Renalase Levels
While we can’t directly supplement renalase (yet), we can support the systems that protect and preserve it:
Reduce Sympathetic Nervous System Activation:
- Practice meditation, deep breathing, or yoga regularly
- Prioritize sleep quality and duration
- Engage in joyful activities – music, art, swimming, dancing
- Address trauma or chronic stress through appropriate therapy
- Consider adaptogenic herbs under practitioner guidance
Support Nitric Oxide Production:
- Consume nitrate-rich vegetables (beets, leafy greens, arugula)
- Consider citrulline or arginine supplementation if appropriate
- Ensure adequate B vitamins for healthy methylation
Manage the RAAS System:
- Work with your healthcare provider on appropriate blood pressure management
- Consider ACE inhibitors or ARBs if prescribed
- Monitor and manage sodium intake appropriately
- Ensure adequate potassium (under medical supervision with kidney disease)
Protect Mitochondrial Health:
- Support with CoQ10, PQQ, or other mitochondrial nutrients
- Reduce oxidative stress through antioxidant-rich foods
- Address any underlying mitochondrial dysfunction
Support Klotho:
- Optimize vitamin D levels
- Manage phosphorus intake appropriately
- Consider bioregulator peptides that support kidney regeneration
Reduce Inflammation:
- Follow an anti-inflammatory diet
- Consider omega-3 fatty acids
- Address gut health and the microbiome
- Manage blood sugar carefully
Consider Bioregulator Peptides:
- Pinealon (adrenal support)
- Pielotax (kidney-specific support)
- Glandokort (adrenal gland bioregulator)
- These oral bioregulators may help support tissue regeneration and function
Conclusion
Renalase represents a crucial but overlooked piece of the kidney disease puzzle. This enzyme connects stress, blood pressure, inflammation, and kidney decline in ways that explain why kidney disease creates such widespread metabolic chaos and why stress accelerates decline. The good news is that understanding these mechanisms gives us multiple intervention points, from stress management to targeted supplementation to working with bioregulators.
While your standard lab work might show “normal” or borderline results, the underlying renalase deficiency may already be creating sympathetic overdrive, oxidative stress, and progressive kidney damage. By addressing the systems that support renalase function, we can work upstream to slow kidney decline, reduce cardiovascular risk, and improve quality of life. This is the essence of integrative, regenerative medicine for kidney disease, not just managing symptoms, but addressing root causes at the cellular and molecular level.