What is fibrosis?
The term fibrosis explains the development of fibrous connective tissue as a response to injury or damage. Fibrosis refers to connective tissue deposition, which occurs as part of the normal healing process, or it can be due to excess tissue deposition, which can occur as part of a pathological process. Scarring is the term we use when referring to fibrosis in response to an injury. Fibrosis can affect any organ or tissue in the body; however, the most common areas are:
And of course, kidneys!
What does fibrosis do to Our Kidneys?
Fibrosis occurs when normally functioning kidney cells are replaced with fibrous connective tissue, similar to scar formation in other parts of the body. It involves an accumulation of excess extracellular matrix, primarily collagen, and usually results in the loss of function when the normal tissue is replaced with scar tissue. Different mechanisms are responsible for kidney fibrosis.
Let’s look at some of the causes!
Causes of Kidney Fibrosis
KD derives from a diverse range of aetiologies, including immunological, mechanical, metabolic and toxic insults…this list is not exhaustive! All of these affect the three compartments of the kidney: the vasculature, glomerulus and tubulointerstitium. It is these compartments that are responsible for the delivery of blood, plasma filtration and modification of glomerular filtrate. Even though matrix synthesis is part of the normal repair process, which occurs after injury, excessive synthesis of the extracellular matrix is destructive and further exacerbates injury, becoming a vicious cycle.
Kidney fibrosis typically develops as a result of:
- Chronic kidney disease (CKD)
- Diabetic nephropathy
- Hypertensive nephrosclerosis
- Glomerulonephritis
- Polycystic kidney disease
- Obstructive nephropathy
- Recurrent kidney infections
How Does Kidney Fibrosis Develop?
We commonly associate scarring with an excessive synthesis of matrix, usually composed of collagen. Keloid scars represent a good example of scarring resulting from abnormal matrix synthesis. Keloids are extreme cases; however, mechanistically, a similar process can occur in organ fibrosis. Fibrosis is a common endpoint in chronic kidney diseases, often resulting from acute kidney injuries (AKIs) or progressive renal insults. The development of kidney fibrosis begins with an initial injury to renal tissue. The injury can result from various causes such as;
- Diabetes
- Hypertension
- Glomerulonephritis
- Exposure to toxins
- Inflammation
- Ischemia
- Obstructive nephropathy
The initial phase of kidney fibrosis occurs following this renal injury. After injury, damaged renal cells release danger-associated molecular patterns (DAMPs). DAMPs activate the innate immune system, triggering an inflammatory response. Inflammation represents the first phase in the cascade of events to follow. Immune cells such as macrophages and dendritic cells become activated, while the circulating inflammatory cells, neutrophils, monocytes and lymphocytes infiltrate the kidney. It is these cells that release the pro-inflammatory cytokines such as interleukin -1β (IL-1β), interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α). This inevitably fuels the inflammatory response, contributing to further tissue damage.
Fibrosis in the kidney: is a problem shared, a problem halved? | Fibrogenesis & Tissue Repair | Full Text
Due to persistent inflammation, conditions become favourable for the activation and proliferation of fibrogenic cells. Multiple cell types contribute to the fibrotic process; myofibroblasts serve as the principal cells responsible for extracellular matrix (ECM) production. Myofibroblasts produce significant amounts of fibronectin, collagen and other ECM components. Dysfunction repair processes can transform the normal wound healing responses into persistent fibrosis. Research shows that transforming growth factor-beta (TGF-β) is a key mediator in fibrosis. TGF-β promotes ECM synthesis and inhibits degradation, all supporting the process of fibrosis.
To add, chronic inflammation plays a central role in sustaining renal fibrosis; cells such as macrophages exacerbate kidney injury and promote fibrogenesis. Studies show inflammatory mediators exacerbate fibrotic responses by driving additional fibrosis through positive feedback, which involves ECM deposition and further cellular activation.
Damaged cells generate excessive reactive oxygen species (ROS) and reactive nitrogen species (RNS). A state of oxidative stress occurs when these free radicals overwhelm the kidney’s antioxidant defence system. Renal vascular undergoes changes that eventually compromise blood flow and nutrient delivery to the kidneys.
As kidney fibrosis continues, scar tissue replaces functional renal parenchyma, leading to the loss of nephron function and the deposition of ECM components leads to tubulointerstitial fibrosis and is closely linked to end-stage kidney disease. It is the balance between ECM production and degradation, coupled with ongoing inflammation, that ultimately dictates whether the kidneys can return to a working state or fall to irreparable damage.
So, what’s next?
Next, what we want to do is slow the process of fibrosis and use agents that will slow the progression of fibrosis. One we need to slow is Transforming Growth Factor-β (TGF-β), which is regarded as the master regulator of fibrosis in organs with a special interest in the kidneys. TGF-β is the strongest stimulus for the activation and proliferation of fibroblasts and their transformation into myofibroblasts, the principal ECM-producing cells in fibrosis. TGF-β drives the progression from acute kidney injury to chronic fibrosis. Research shows that targeting the TGF-β pathway provides a promising approach towards fibrosis in chronic kidney disease.
Why is TGF-β a Problem?
When the kidney is injured due to diabetes, hypertension, infection, etc, TGF-β is released and activated. Usually, it is in its inactive form; however, with injury, it becomes activated.
Once active, TGF-β transforms various kidney cells into fibrosis-producing cells:
- Kidney fibroblasts become activated and turn into myofibroblasts.
- Tubular epithelial cells may partially transform, losing their normal function
- Endothelial cells in blood vessels can convert to matrix-producing cells
These transformed cells, especially myofibroblasts, are what produce scar tissue.
Once scar tissue is produced, a self-perpetuating cycle begins. Scar tissue stores more TGF-β, stimulating its own production. An increase in tissue stiffness activates more TGF-β, further exacerbating the issue.
As this cycle continues, normal kidney tissue is gradually replaced by scar tissue, leading to:
- Loss of functioning nephrons
- Reduced filtering capacity
- Decreased kidney function
- Kidney failure ensues if the process isn’t stopped
Once established, it is a condition difficult to reverse.
And this is where peptides can step in!
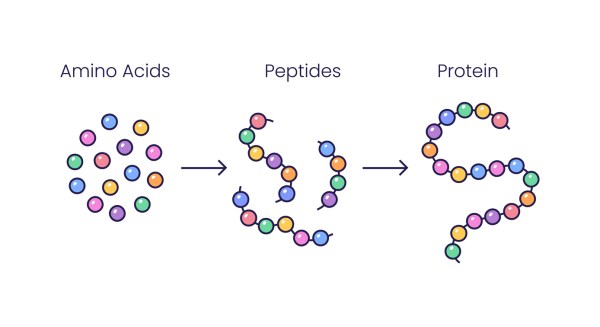
What Are Peptides?
Peptides represent small chains of amino acids, typically containing fewer than 20 of these protein building blocks linked together. When amino acid chains extend beyond this threshold of 20 units, they are generally classified as proteins. Proteins have more complex structures and functions. In the body, peptides serve as molecular messengers, facilitating intercellular communication that promotes cellular healing, extends cellular longevity, and enhances overall cellular performance. Their ability to signal like this enables peptides to initiate repair processes throughout the body. As a therapeutic agent, peptides send signals that interact with target cells. They can substitute for naturally occurring peptides or mimic their actions, modifying the body’s biochemical processes. This communication allows peptides to stimulate regenerative processes and support tissue reconstruction.
B7-33
B7-33 is a synthetic peptide, a derivative of relaxin, derived from the B-chain of human relaxin-3 (also known as INSL7 or RLN3), comprising amino acids 7-33 and the B-chain. This specific peptide is less well-known; however, it has gained attention in the research world due to its individual actions and therapeutic potential. As previously stated, a major mediator in renal fibrosis is TGF-β, which activates downstream pathways involving Smad2 and Smad3. This leads to increased collagen deposition and fibrotic changes in kidney tissue. Research has found that inhibiting the signalling pathways of TGF-β can protect against renal fibrosis. Research has indicated that B7-33 can reduce collagen thickness and matrix metalloproteinase-2 (MMP-2) activity, which is essential for collagen degradation.
Interleukin-33 (IL-33) in renal fibrosis is also gaining recognition. Increased IL-33 levels have been associated with inflammatory responses leading to ischemia-reperfusion injury (IRI) and then fibrosis. IL-33 and TGF-β are therefore important mediators in renal fibrosis, offering hope for more effective interventions.
For kidney damage related to hypertension, B7-33 may support the body by;
- Preventing vascular sclerosis
- Reducing interstitial fibrosis
- Protecting against epithelial-to-mesenchymal transition
B7-33 shows vasodilatory effects similar to those of full-length relaxin, these include;
- Vasodilation through nitric oxide-dependent mechanisms
- Reduction of vascular stiffness
- Angiogenic properties that may promote tissue repair
Studies show B7-33 may improve vascular function by promoting angiogenesis and reducing endothelial cell apoptosis, supporting the process of vascular repair.
KPV Peptide & Inflammation
The tripeptide KPV (lysine-proline-valine), has shown significant anti-inflammatory properties. Inflammation is one of the key drivers in kidney fibrosis and drives its progression; it holds promise in cases of inflammation and kidney fibrosis. KPV has shown anti-inflammatory effects in vivo and in vitro via modulating immune cell responses. KPV has been associated with protection against other inflammatory conditions, especially intestinal inflammation, suggesting a systemic action where modulating inflammation in one organ may benefit other organs. As cytokines such as TNF-α and IL-6 are important mediators of renal inflammation, KPV’s ability to control their production could mean it shows protective effects against renal fibrosis.
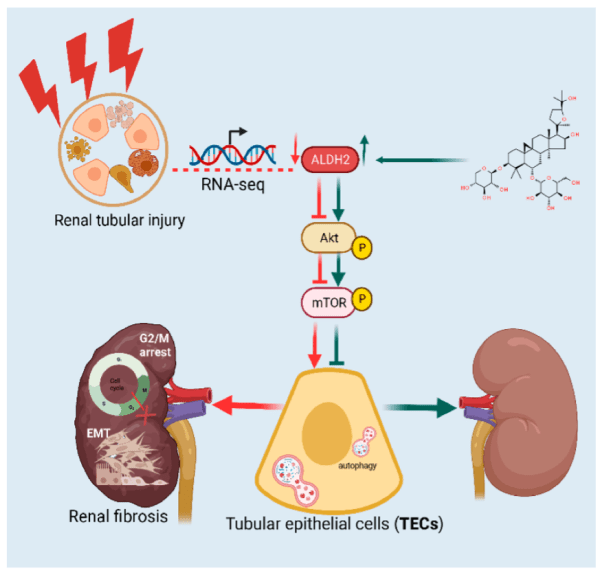
SS-31 & Renal Fibrosis
SS-31 is a mitochondria-targeted tetrapeptide that has gained interest for its protective effects against oxidative stress-induced tissue damage. SS-31 can repair mitochondrial dysfunction. It has antioxidant properties; therefore, it scavenges reactive oxygen species (ROS) within mitochondria, preventing oxidative damage to renal tubular cells. SS-31 has been found to decrease the levels of pro-inflammatory cytokines and inhibit pathways involved in cellular death. Its role in targeting renal fibrosis role of SS-31 is primarily attributed to its antioxidant properties, ability to modulate inflammatory pathways, and protective effects on renal cellular structures.
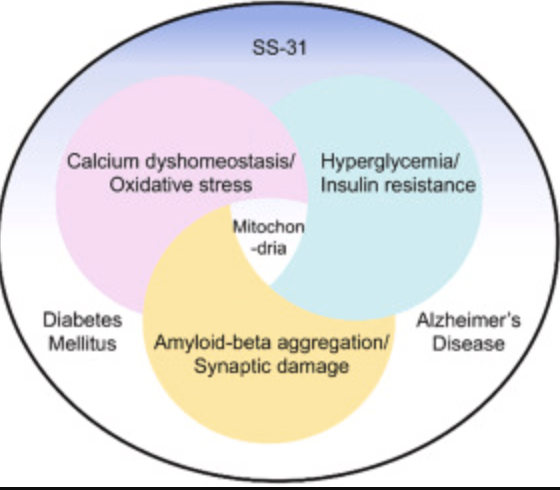
Image: https://www.sciencedirect.com/science/article/abs/pii/S1043661821003674
Peptides hold promise as therapeutic agents for preventing or treating kidney fibrosis through mitochondrial protection, reducing inflammation and oxidative stress, and restoring cellular homeostasis. Peptides’ ability to address many of the pathological mechanisms makes them suited to the complex, self-perpetuating cycles of kidney fibrosis.
The Promise of Peptides in Kidney Fibrosis Treatment
Kidney fibrosis represents a significant challenge in nephrology, affecting millions worldwide as a common endpoint for various kidney diseases. As explored throughout this article, the complex pathophysiology of renal fibrosis—involving inflammation, oxidative stress, cell transformation, and excessive ECM deposition—makes it particularly difficult to treat using conventional approaches.
Peptide therapies such as B7-33, KPV, and SS31 offer a revolutionary approach to combating kidney fibrosis by targeting multiple pathways simultaneously. These innovative treatments address the root causes of fibrosis rather than merely managing symptoms:
- B7-33 helps counteract TGF-β signalling, reducing collagen deposition while supporting vascular health and repair
- KPV peptide modulates inflammatory responses that drive fibrosis progression
- SS31 protects mitochondrial function and reduces oxidative stress, preserving kidney cellular integrity
The multifaceted action of these peptides makes them uniquely positioned to break the self-perpetuating cycle of kidney fibrosis. By simultaneously addressing inflammation, oxidative damage, and matrix deposition, peptide therapies could potentially slow, halt, or reverse fibrotic processes that have traditionally been considered irreversible.
For patients suffering from chronic kidney disease, diabetic nephropathy, or other conditions leading to kidney fibrosis, these emerging peptide treatments represent hope beyond conventional therapies. As research advances, we may soon see personalised peptide protocols that target specific aspects of kidney fibrosis based on individual patient needs and disease mechanisms.
With these peptide innovations, the future of kidney fibrosis treatment looks promising. By preserving kidney function and potentially reducing the progression to end-stage renal disease, peptide therapies could dramatically improve the quality of life for kidney disease patients while reducing the enormous healthcare burden associated with kidney failure.
If you or a loved one is dealing with kidney disease, consult with a nephrologist about the latest treatment options and clinical trials exploring these cutting-edge approaches to managing kidney fibrosis.
Note: This article is for informational purposes only and does not constitute medical advice. Always consult with qualified healthcare professionals regarding treatment options for kidney disease or any medical condition.